What are biologic medicines?
A biologic is an active pharmaceutical ingredient derived from a living organism that cannot be synthesized by chemical means.1

Recombinant proteins
Through better understanding of pathologic pathways, recombinant proteins such as glycosylated monoclonal antibodies (mAbs) and fusion proteins have emerged as therapeutic candidates to treat many diseases because of their specificity and stability in binding to molecular, pharmacological targets (Examples).2
These therapies have greatly advanced treatment paradigms and improved outcomes of many diseases since their first introduction in the 1990s. However, they are, in the vast majority of cases, more expensive than conventional small-molecule medications and broad use can significantly impact healthcare budgets. As a result, access to these therapies is frequently limited through the application of reimbursement criteria that are often stricter than treatment guidelines potentially leading to sub-optimal treatment of eligible patients.2,3
Biologics have large and complex structures compared to small-molecule drugs. Unlike small-molecule drugs, which are chemically defined molecular entities, biologics such as recombinant monoclonal antibodies and fusion proteins have greater structural complexity, including primary, secondary, tertiary and quaternary structures. They can be more than 1000-fold larger than small-molecule drugs.9,10
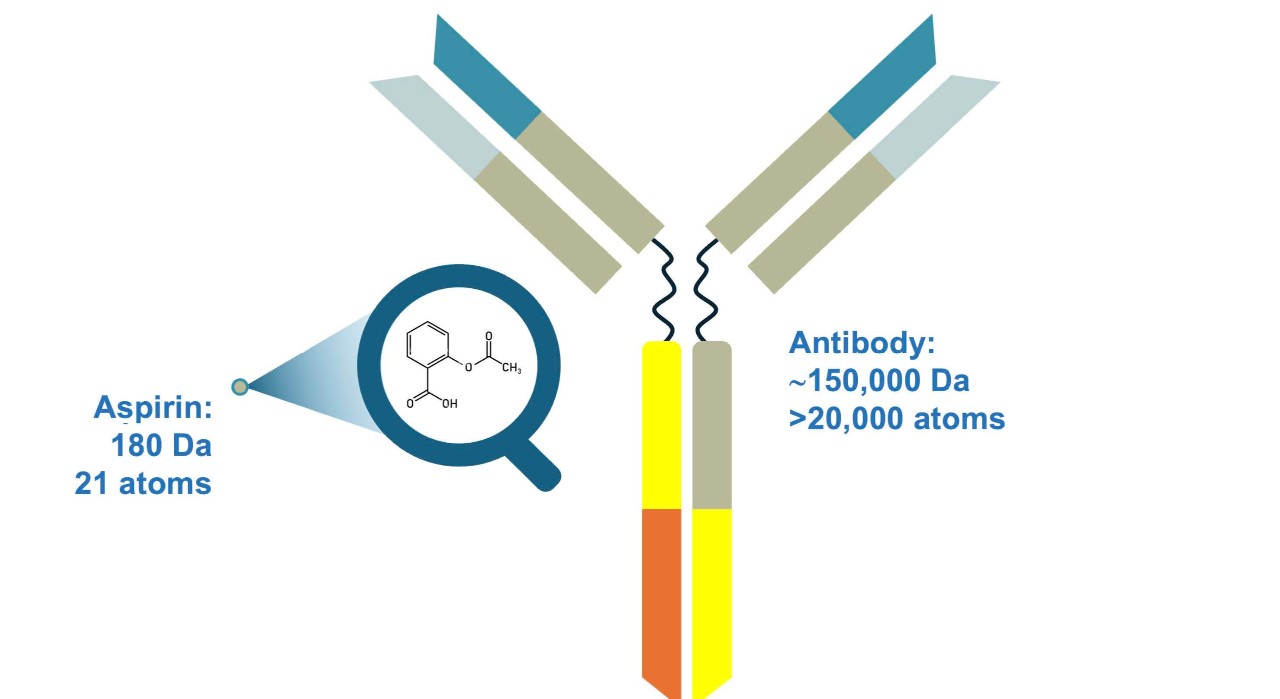

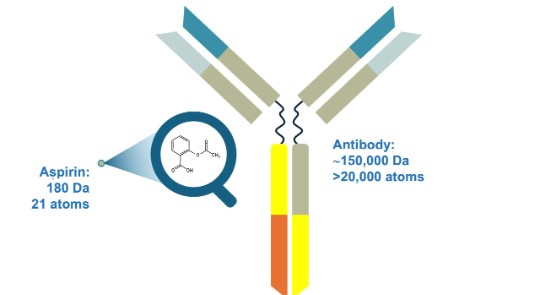
Adapted from Kozlowski S, et al. N Engl J1 Med 2011;365(5):385–388.
Variability is an inherent characteristic of biologics
Recombinant proteins are engineered by introducing manipulated DNA into a living organism (cell lines) such as bacteria (E. coli), yeast (ex. S. cerevisiae), mammalian (ex. CHO, Chinese Hamster Ovary), or human cells (ex. HEK293, HT-1080). The DNA instructs the living organism to produce large quantities of the recombinant protein.11
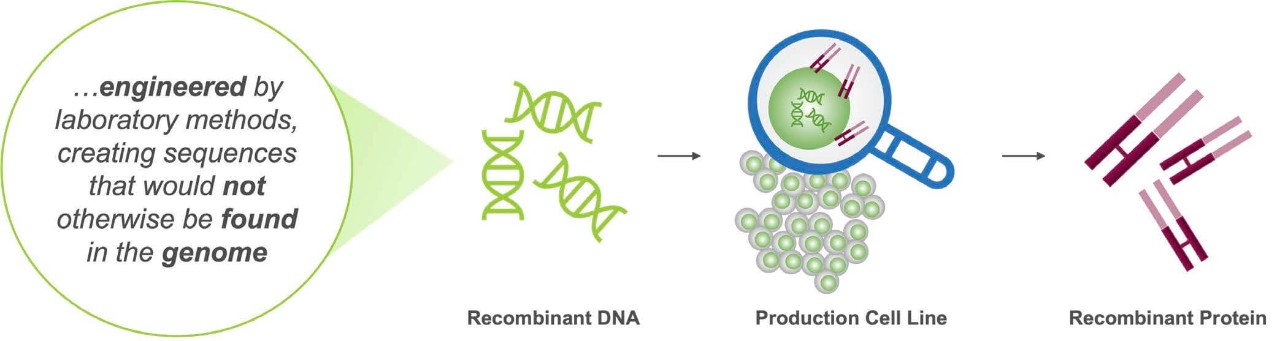

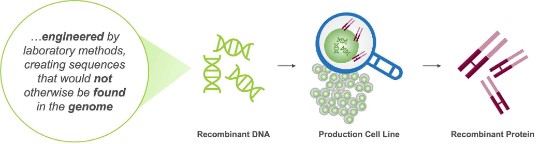
DNA, deoxyribonucleic acid.
Icons designed by Freepik from Flaticon (https://www.flaticon.com/).
The biologic activity of recombinant proteins is defined by their complex structure but also by the cell-line involved in the manufacturing process to produce them.10-12 For instance, post-translational modifications such as glycosylation pattern but also charge profile are very dependent on the cell line used for manufacturing and can be defined as critical or non-critical quality attributes (CQA) for the biological activity of a recombinant protein. This is also called the “biochemical fingerprint” of a protein. It defines its pharmacological activity and ultimately its efficacy, safety profile and immunogenicity.12
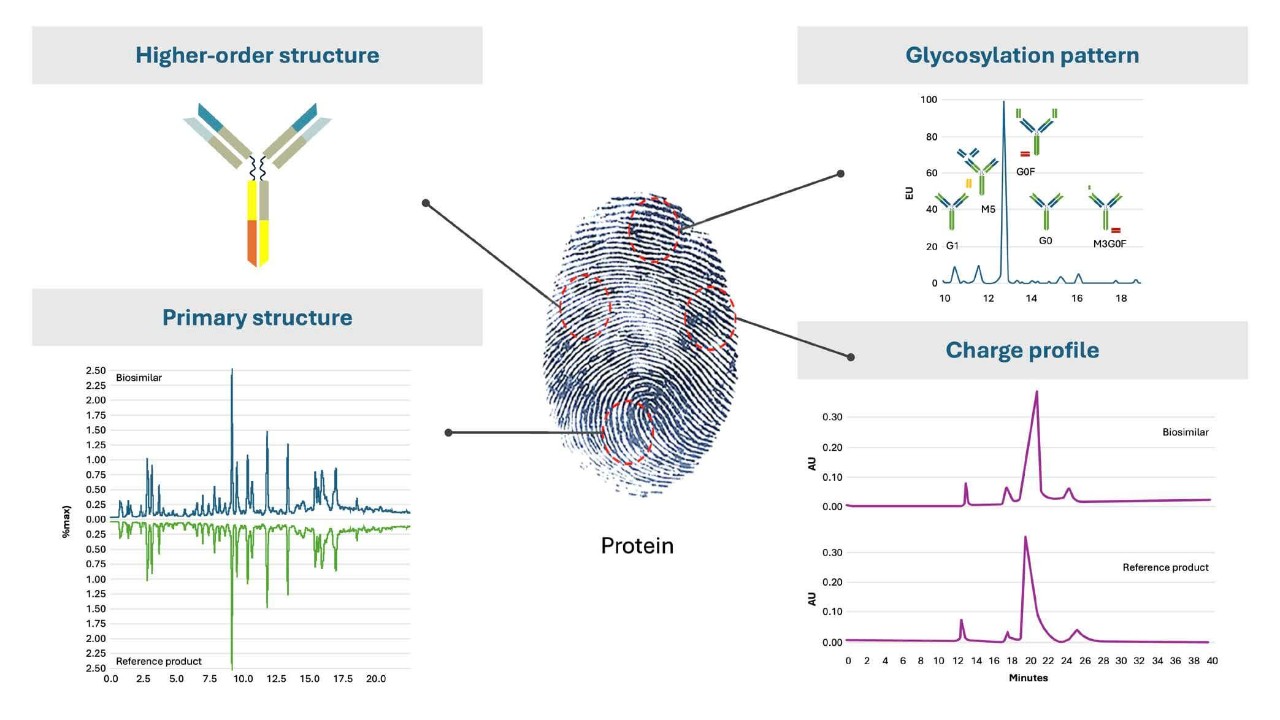

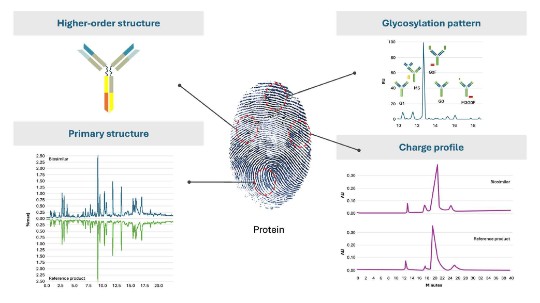
Images shown are for illustrative purposes only, and are adapted from the below mentioned references.
1. Berkowitz S, et al. Nat Rev Drug Discov 2012;11(7):527–540; 2. Lee C, et al. MAbs 2017;9(6):968–977;3. Turner A & Schiel JE. Anal Bioanal Chem 2018;410(8):2079–2093; 4. Cho IH, et al. MAbs 2016;8(6):1136–1155.
There is an inherent variability during the manufacturing of recombinant proteins. Manufacturing follows a complex multistep process, and each step can impact the quality of the product:
Living organisms modify the recombinant proteins they produce. This variability can be:
- 1) Chemical modifications
- 2) Biosynthetic or enzymatic modifications
Some modifications occur more often, others less frequently, resulting in a distinct spectrum of variants that can be measured and reproduced.14
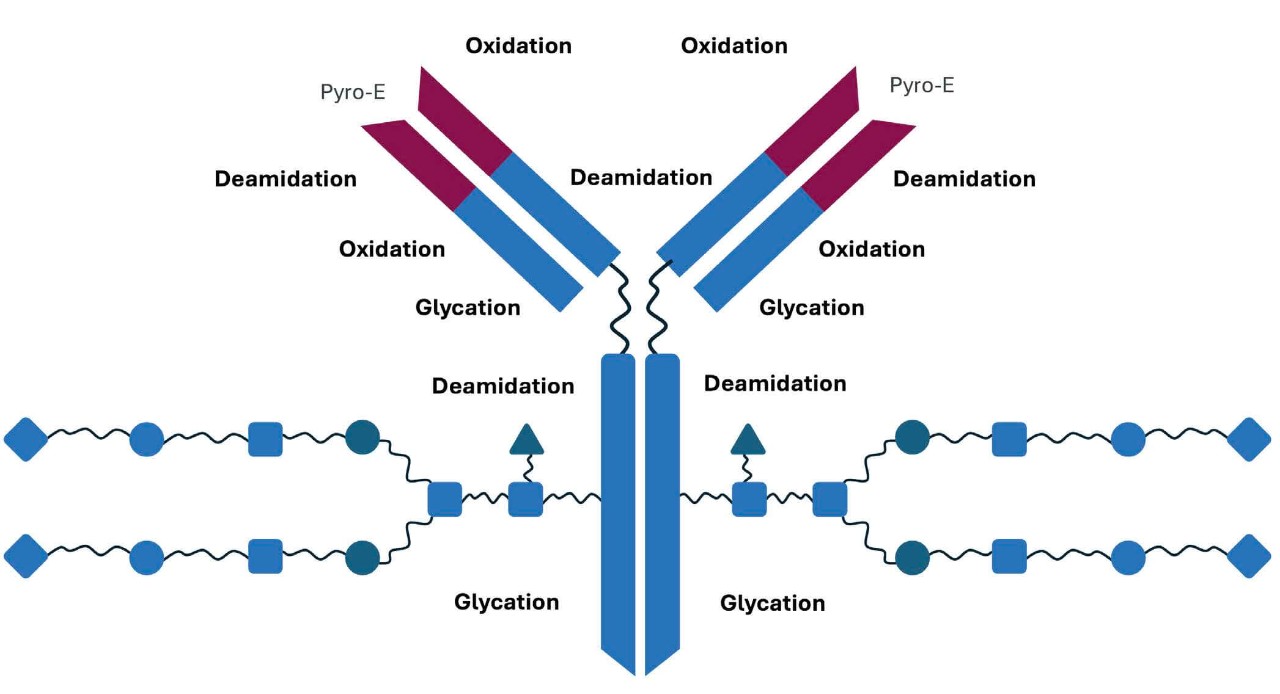

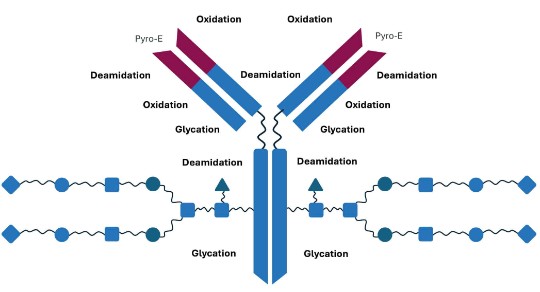
Adapted from Kozlowski S & Swann P. Adv Drug Deliv Rev 2006;58(5-6):707–722.
Hence, no two lots are the same even when the manufacturing process is “unchanged”, which makes them impossible to be replicated exactly.12,15,16
Further, there are numerous post-approval manufacturing changes observed in monoclonal antibodies (see Figure below).17
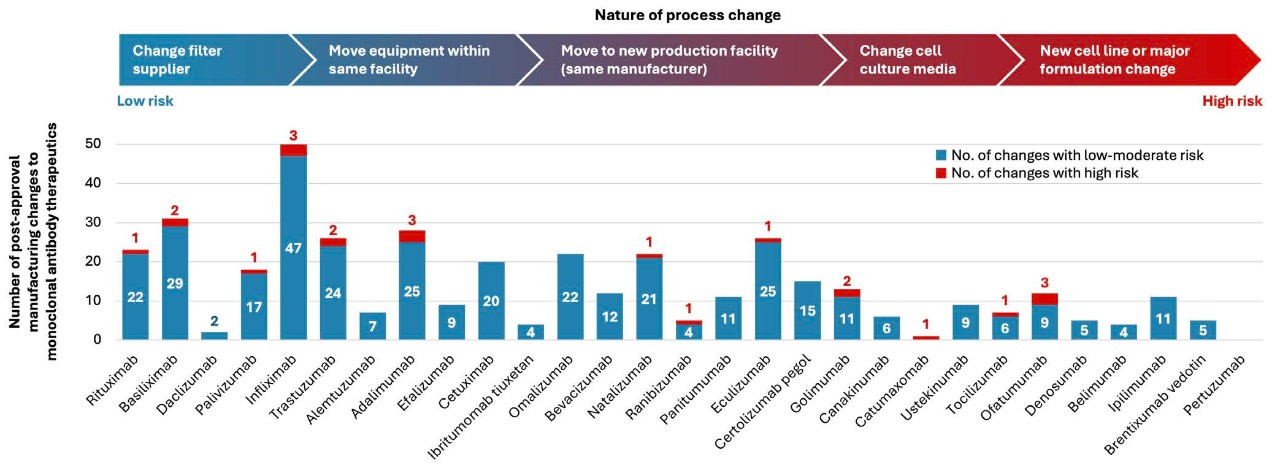

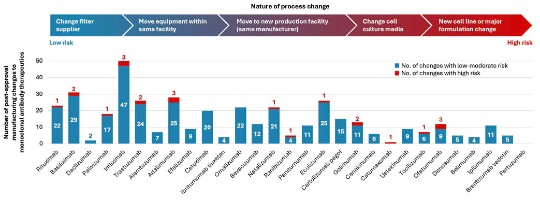
Figure 1. The biologics manufacturing process and the manufacturing steps that affect final characteristics of biologics. Adapted from Vezér B, et al. Curr Med Res Opin 2016;32(5):829-834.17
By the International Council for Harmonisation (ICH) of Technical Requirements for Pharmaceuticals for Human Use, product changes introduced through manufacturing changes should have no adverse impact on critical quality attributes (“biological fingerprint”). If there are changes, additional data and studies are required for risk minimization.24
1.Revers L, Furczon E. CPJ/RPC 2010;143(4):184–191.
2.Baumgart DC, et al. Front Pharmacol. 2019;10:279.
3.Putrik P, et al. Ann Rheum Dis. 2014;73(1):198–206.
4.Smolen JS, et al. Ann Rheum Dis. 2020 Jun;79(6):685-699
5.Turner D, et al. Gastroenterology. 2021;160(5):1570-1583.
6.Romero IB, et al. Ann Med 2021;53(1):1727-1736.
7.Augustin M, et al. Dermatol Ther (Heidelb) 2024;14(10):2841-2847.
8.Finger RP, et al BMC Ophthalmology 2020;20(1):294.
9.Abraham J, et al. Semin Oncol 2013;40(Suppl. 1):S5–S24.
10.Kozlowski S, et al. N Engl J Med 2011;365(5):385–388.
11.Kantardjieff A, Zhou W. Adv Biochem Eng Biotechnol. 2014:139:1-9.
12.Vulto AG & Jaquez OA. Rheumatology (Oxford) 2017;56:iv14–iv29.
13.Vandekerckhove K, et al. AAPS J. 2018:20(4):68
14.Kozlowski S & Swann P. Adv Drug Deliv Rev 2006;58(5-6):707–722.
15.Ramanan S & Grampp G. BioDrugs 2014;28(4):363–372.
16.Schiestl M, et al Nat Biotechnol 2011;29(4):310-312.
17.Vezér B, et al. Curr Med Res Opin 2016;32(5):829–834
18.WHO. Good manufacturing practices. 2015. https://www.who.int/news-room/questions-and-answers/item/medicines-good-manufacturing-processes, accessed January 2025.
19.MHRA. Good manufacturing practice and good distribution practice. 2014. https://www.gov.uk/guidance/good-manufacturing-practice-and-good-distribution-practice, accessed January 2025.
20.EMA. Good manufacturing practice. 2008. https://www.ema.europa.eu/en/human-regulatory-overview/research-development/compliance-research-development/good-manufacturing-practice#inspections-for-pharmaceutical-startingmaterials-section, accessed January 2025.
21.FDA.gov. Facts About the Current Good Manufacturing Practices (CGMP). 2024. https://www.fda.gov/drugs/pharmaceutical-quality-resources/facts-about-current-good-manufacturing-practice-cgmp, accessed January 2025.
22.WHO. WHO good manufacturing practices for biological products., Annex 2, TRS No 999. 2016. https://www.who.int/publications/m/item/annex-2-trs-no-999-WHO-gmp-for-biological-products, accessed January 2025.
23.ICH. ICH harmonisation for better health. 2023. https://www.ich.org, accessed January 2025.
24.ICH Topic Q5E 2005. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-5-e-comparability-biotechnologicalbiological-products-step-5_en.pdf, accessed January 2025.
Abbreviations:
Learn more
Read more about biologics and biosimilars in the following publications:
Patel P, et al, 2015, J Dermatolog Treat;6(4):299-302.
Biologics and biosimilars
Biological drugs are large, complex glycoprotein molecules produced in living organisms that have revolutionized the treatment of many conditions. As biologics used in several therapeutic areas will face patent expiration, this opens opportunities for competitive versions (biosimilars). As biologics are complex molecules they can never be replicated exactly; even innovator biologics have inherent batch-to-batch variability. When the second batch of innovator products were released, physicians began prescribing non-identical variants of biologics to their patients, accepting the possibility of variation in clinical effects. Unlike the variants in innovator products, biosimilars will provide clinical trial data demonstrating similar clinical effects, though there will always be some degree of uncertainty in how much clinical impact will be result from the variation in both innovator and biosimilar products.
Chan JCN & Chan ATC, 2017, ESMO Open. 2017; 2(1): e000180.
Biologics and biosimilars: what, why and how?
Despite the high cost of development, due to their targeted nature with high efficacy, biologics are now taking on an increasingly important role in the treatment of common and/or serious diseases such as diabetes, cancer, chronic kidney disease, rheumatoid arthritis, psoriasis, blood disorders, vaccines and inflammatory bowel diseases. Unlike single molecules which are chemically synthesised with highly predictable structures and functions, biologics are pharmaceutical compounds synthesised or extracted from a biological source often with highly complex structures.